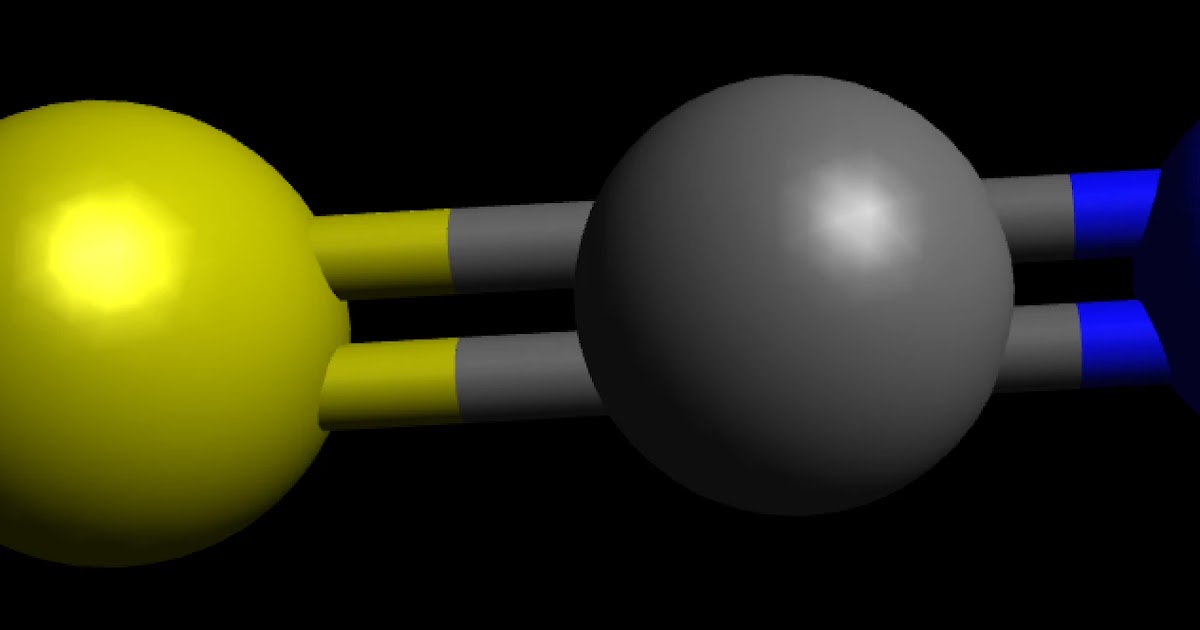
To determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. In the case of thiocyanate ion. So, is scn polar or nonpolar?
SCN Lewis Structure How to Draw the Lewis Structure for SCN
How to tell if a lewis structure is polar?
To determine if a lewis structure is polar, examine the molecular geometry and bond polarity.
Bond polarity and molecular polarity are different (though related) concepts. Scn ion is polar because of the unequal electronegativity of sulfur(2.58), carbon(2.55), and nitrogen(3.04). Its linear structure makes it a. Given a pair of compounds, predict which would have a higher.
In short the molecular dipole moment is the vector sum of the individual bonds dipole moments, so you. This is because the bonds of the compounds have an imbalance in the position of the electrons. Determine if a molecule is polar or nonpolar. Explain how polar compounds differ from nonpolar compounds.
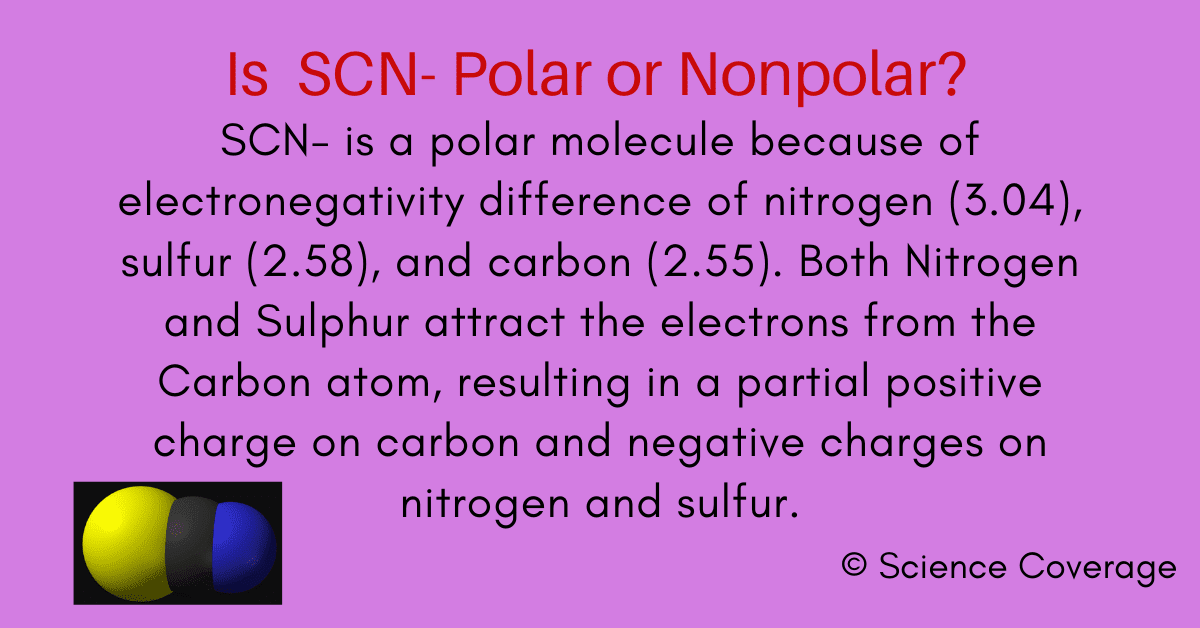
And also, as there is a.