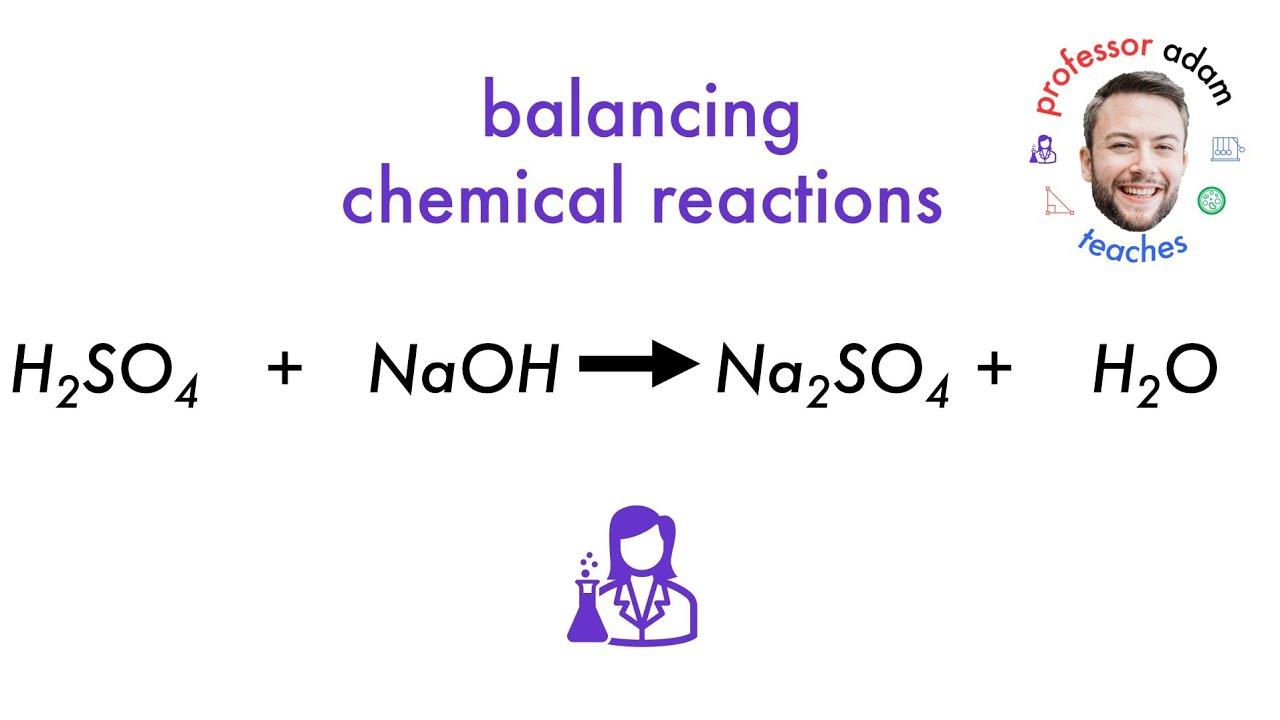
H2so4 is an acid and naoh is a base.so here acid and base. In a neutralization reaction, an acid reacts with a. 2naoh + h2so4 → na2so4 + 2h2o
CHEMISTRY RESOURCE 9C ACIDS, BASES and SALTS ppt download
The question is asking about the reaction of sulfuric acid (h2so4) with sodium hydroxide (naoh) to determine which salt is produced from this neutralization reaction.
In this reaction salt and water are always the products of the reaction.
There was an issue loading this video. Therefore, the correct answer is na2so4. When h2so4 reacts with naoh, it forms h2o and na2so4. Since the base and acid are strong the reaction goes to.
When sulfuric acid (h 2s o4) reacts with sodium hydroxide (naoh), they undergo a neutralization reaction. To determine which salt is formed in the neutralization reaction between h x 2 s o x 4. When sulfuric acid (h\(_2\)so\(_4\)) reacts with sodium hydroxide (naoh), a neutralization reaction occurs, producing a salt and water. Naoh + h2so4→ na2so4 + h2o.

Here's how the reaction proceeds step by step:
Neutralization reactions occur between an acid and a base to produce a salt and water. Answer:when h2so4 reacts with naoh,the salt na2so4 (sodium sulphate) is produced. It is observed that active metals like zinc react with strong bases like. In this reaction, an acid and a.
Name the gas which will be evolved when the same metal reacts with dilute solution of a strong acid. The type of chemical reaction that occurs when h2so4 react with naoh is neutralization reaction; When h2so4 (sulfuric acid) reacts with naoh (sodium hydroxide), it forms na2so4 (sodium sulfate) and h2o (water). The balanced chemical equation for this reaction is:
The reaction between a strong acid and a strong base results in salt and water.
When sulfuric acid (h 2s o4) reacts with sodium hydroxide (n aoh), it is a type of reaction known as a neutralization reaction. The salt produced when h2so4 reacts with naoh is sodium sulfate (na2so4). The salt produced is n a 2 s o 4. The balanced equation for the reaction between sulfuric acid and sodium hydroxide is consistently represented as h 2 s o 4 + 2 naoh → na 2 so 4 + 2 h 2 o, which confirms.
) is a strong acid. When sulfuric acid (h₂so₄) reacts with sodium hydroxide (naoh), a neutralization reaction occurs, which is a fundamental concept in chemistry.


+%2B+2NaOH(aq)+%EF%83%A7%3D%3D%3D%3D%3D%3D%EF%83%A8+Na2SO4(aq)+%2B+2H2O(l).jpg)