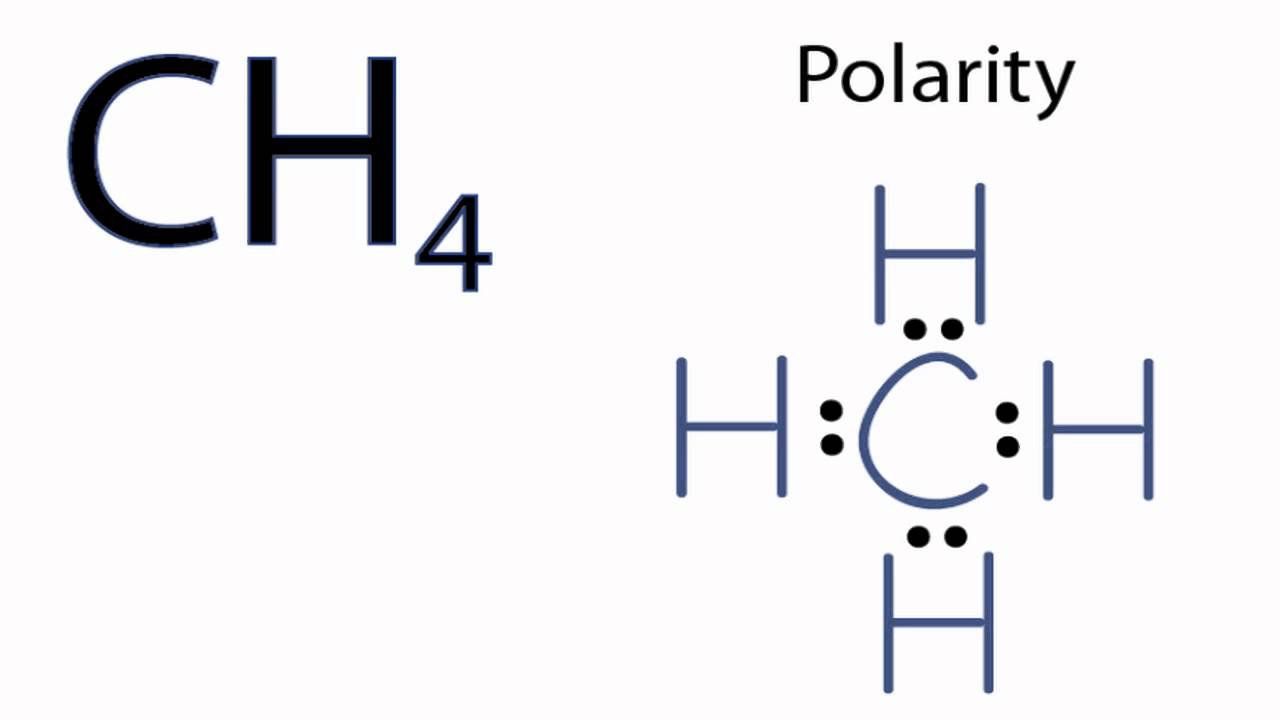
Is p4 polar or nonpolar? For example, symmetric molecules, like co 2, bf 3, and ccl 4, are nonpolar, although each bond in them is polar. The dipole moment measures the extent of net.
Is P4 Polar Or Nonpolar? The 13 Detailed Answer
P4 is a nonpolar molecule because it has a symmetric tetrahedral shape with four identical phosphorus atoms and there are no lone pairs on the central atom, leading to no net.
Molecular geometry, dipole moment, and electronegativity.
The electrogativity difference gives the magnitude and direction of polarity across each bond since the arrow always points to the. Since all of the atoms in the bond are the same, there is no polarity in the bonds between the atoms, so the entire molecule is non polar. It only has homoatomic bonds and on top of that it is. There will be no polarity between in the bonds between the atoms, and thus no polarity in the compound as.
If such a charge separation exists, the molecule is said to be a polar molecule (or dipole); For example, pb(zr, ti)o 3 (pzt) has two stable polar states with the same energy and magnitude of polarization 27. See answer (1) best answer. Is p4 a polar molecule.

In p4, all of the atoms bonding are the same.
However, more complex behaviors have been identified in. There will be no polarity between in the bonds between the atoms, and thus no polarity in the compound as. Molecules are two or more atoms. In p4, all of the atoms bonding are the same.
When the difference is small, the bond is nonpolar. To determine whether tetraphosphorus (p 4) is polar or nonpolar, we can examine it from three key perspectives: P4 is nonpolar because the 4 p atoms are arranged about a center of symmetry and they have the same electronegativity. Is p4 polar or nonpolar?

3.8.5 illustrates the symmetric molecules that have polar bonds, but the.
Otherwise the molecule is said to be nonpolar.