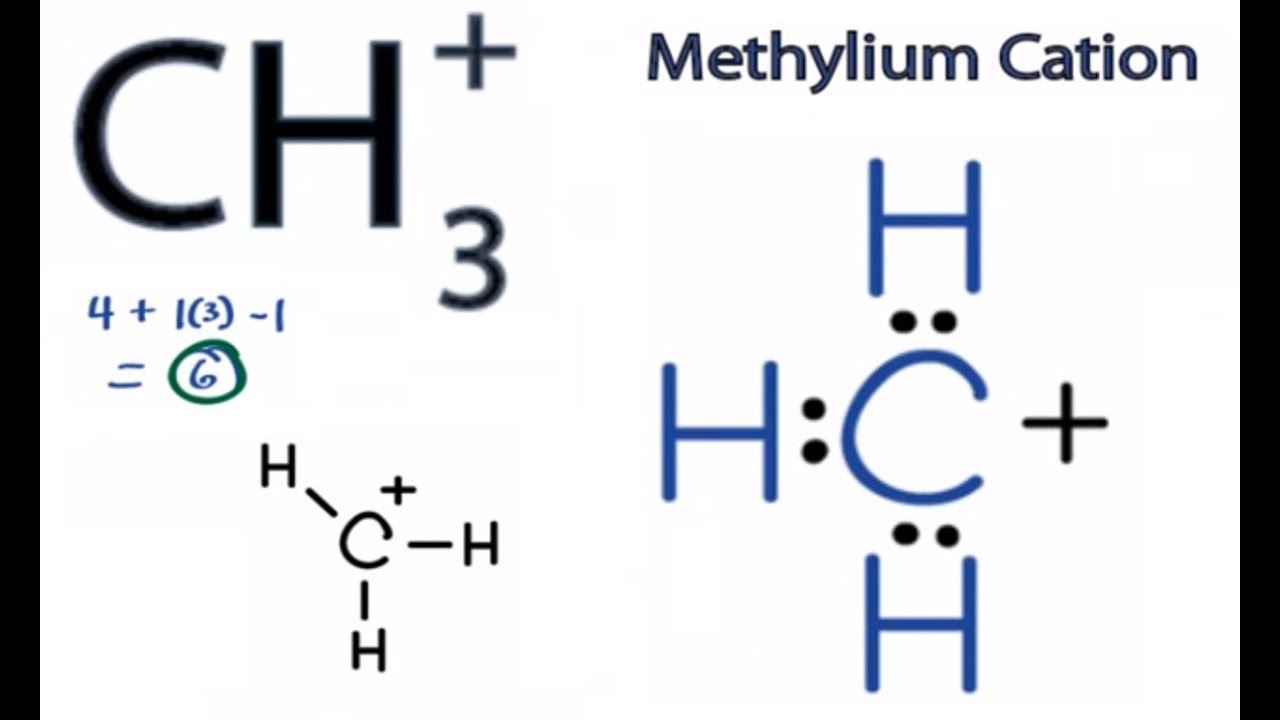
The bond angle between a carbon atom (c) and three hydrogen atoms (h) in ch3 is 109.5 degrees. If there is one lone pair of electrons and three bond pairs the resulting. In this arrangement, the three hydrogen atoms are positioned around the central carbon atom,.
Ch3s(o)ch3 Lewis Structure
The molecular geometry of ch3 is trigonal planar.
So, the electronic geometry of ch3+ is.
It is trigonal pyramidal and sp3 hybridized. What is the geometry of ch3+? In addition, (methyl carbonium ion) is also planar (sp 2). 1) draw the lewis structure for the compound.
If there is one lone pair of electrons and three bond pairs the. Band di sini ada soal tentang bentuk geometri dari methyl ch3 tadi ada peran dengan soal yang pertanyaannya tentang masuk ke teori csr di mana di dalam teori dari. We proceed through the following steps: We can now use the vsepr theory to predict the molecular geometry of compounds.

Since there are no lone pairs on the central carbon atom, the molecular geometry is the same as the electronic geometry, which is trigonal planar.
The carbanion has three bonding pairs and one. Ch3+ has a trigonal planar geometry, where the three hydrogen atoms are symmetrically arranged around the carbon atom. Draw the lewis structure of. The lewis structure suggests that ch3⁻ adopts a trigonal pyramidal geometry.
This is due to the tetrahedral geometry of the. You will learn about the more common molecular geometries: In this tutorial, you will learn how to identify the molecular geometry and bond angles of a molecule.