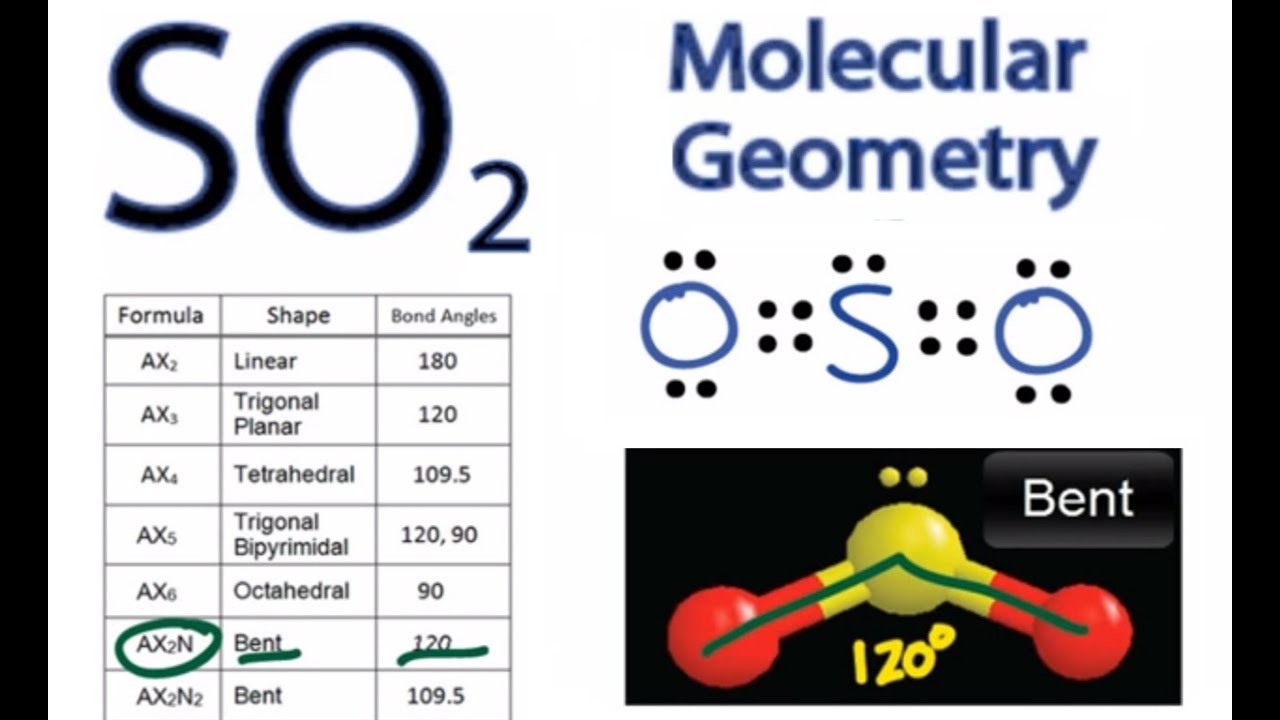
Label the diagram by dragging the labels to the appropriate targets. So molecule is consisted of a sulfur atom surrounded by two oxygen atoms bonded by two double bonds and one lone pair. Labels can be used once, more than once, or not at all.
SO2 Molecular Geometry / Shape and Bond Angles (Sulfur Dioxide) YouTube
Drag the appropriate labels to their respective targets.
Drag the appropriate labels to their respective targets.
Covalent bonds are caused by two atoms sharing their. Label all bonds in so label the diagram by dragging the. Drag the appropriate labels to their respective targets. In the sketch of the structure of so2 label all bonds.
In the structure, each oxygen atom is attached to sulphur by an σ and a π bond. Note that not all labels will be used. Drawing the lewis structure for so2 is essential for understanding its molecular bonding and chemical properties. Labels can be used once, more than once, or not at all.

To label the bonds in each of the given molecules (ch2br2, so2, nf3, bf3), we need to identify the types of bonds present in each molecule.
Labels can be used once, more than once, or not review constants periodic table in the sketch of the structure of so, label all bonds. Solution for in the sketch of the structure of so2, label all bonds. Label the diagram by dragging the labels to the appropriate targets. S and o atoms have sp2.
Labels can be used once, more than once, or not at all: Label all bonds in so2. Labels can be used once, more than once, or not at all. There are 2 steps to solve this one.

A sulfur atom (s) and two oxygen atoms (o) make up the so2 lewis structure.
Sulfur dioxide lewis structure is drawn step by step using vespr rules. In the sketch of the structure of so2 label all bonds. In the lewis structure, the dash represents chemical bonds, and the dots represent lone pairs. Label all bonds in so2.
The sulfur atom (s) is the center atom, and the two oxygen atoms (o). In the sketch of the structure of bf3, all bonds are labeled correctly. Label all bonds in so2 .label the diagram by dragging the labels to the appropriate targets. In so2 lewis structure, there are two double bonds between sulfur atom and oxygen atoms.

Drag the appropriate labels to their respective targets.
The labels for the bonds are. Vsepr theory can predict the shape of the sulfur dioxide molecule. To correctly label the bonds in the structure of so2, we need to consider the hybridization of the sulfur atom and the orbital overlap between sulfur and each oxygen atom. Your solution’s ready to go!
Drag the appropriate labels to their respective targets. Hybridization is a phenomenon of mixing of atomic orbitals of an atom to form hybrid orbitals.