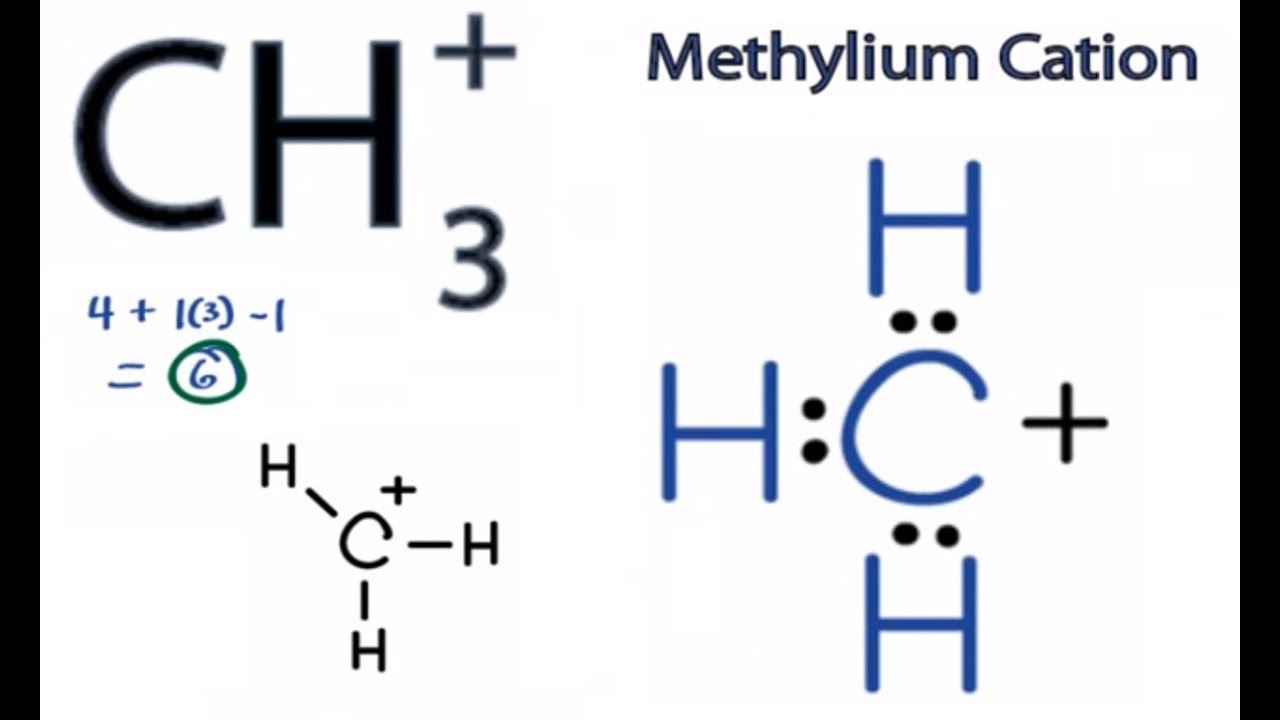
But in ch 3, , a positive charge makes the number of available electrons 3. Find more chemistry widgets in wolfram|alpha. Keeping hybridisation of carbon atom in mind, identify the molecule which is linear:
CH3+ Lewis Structure Methyl cation YouTube
The shape of cf_3 is pyramidal unlike ch_3.
What is a methyl group (ch3)?
It forms 3 single bonds with h to form a planar structure with sp2 hybridization. The carbanion has three paired bonds and one lone pair. Note that typically for lewis structures brackets are placed. There are 3 single bonds between the carbon atom (c) and each.
A methyl group, denoted by the symbol ch3, is a functional group consisting of one carbon atom bonded to three hydrogen atoms. Planer or pyramidal is the shapes of alkyl free radical from the evidence of. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Hence, the correct answer is.
Methyl was discovered in interstellar medium in 2000 by a team led by.
![[CH3]](https://i2.wp.com/www.chemtube3d.com/images/gallery/inorganicsjpgs/ch3_-.jpg)


